
And now we need to determine the mass of chromium trioxide that is record. It's equal to the number of moles of chromium three self right? So it is 4.52 moles here. So we can definitely compute that the number of moles of chromium trioxide that will be required. So we observe that One mole of chromium three cell fight is produced from one mole of chromium trioxide. This proves that the equation is balanced here. And here also we have three oxygen atoms. Also, we have to chromium atoms three sulfur atoms on the left, three on the right, six hydrogen and left six hydrogen to the right three oxygen atoms. In fact to chromium atoms on the right side. Is it balanced or not? So on the left hand side we have two molds off. So that's the number of moles of chromium three cell fight. So here we simplify, we divide 904 With 200 and we get four points 50 miles. So we know that number of moles that's equal to mass per unit molar mass. So let's figure out the number of moles of this chromium three self fight.
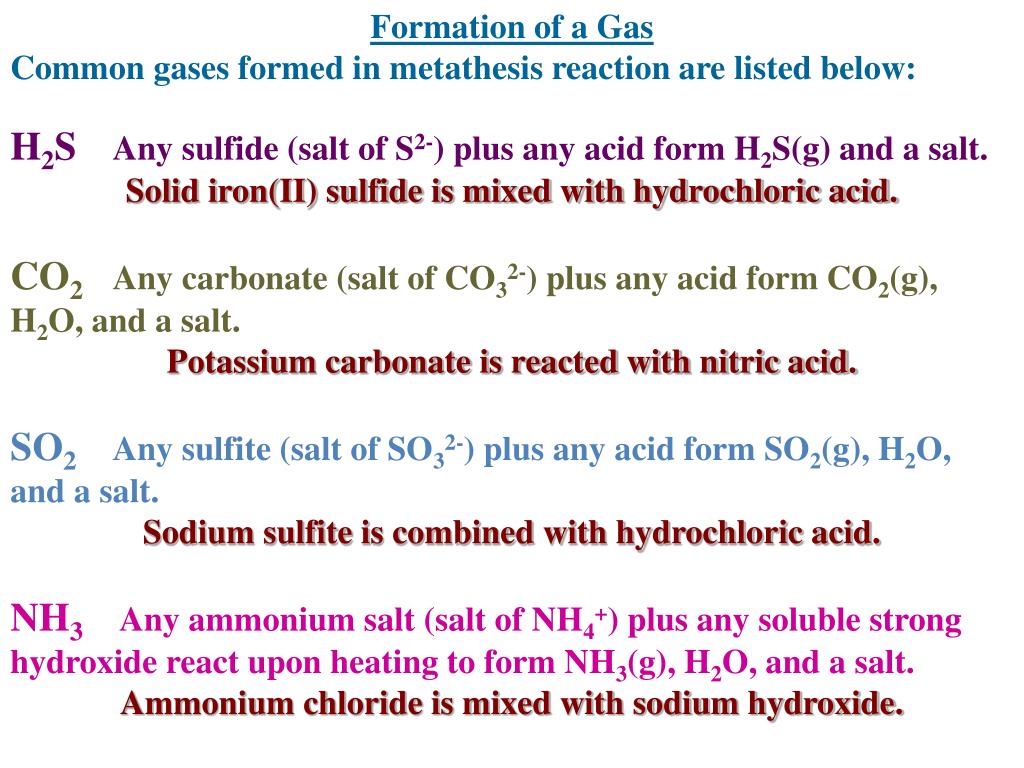
#Chromium iii sulfide plus#
R to S three, the Mueller must will be Two times of chromium, that's 104 plus three times of sulfur.
So we'll get 34 g per mole as the molar mass of this compound. And the molar mass for hydrogen sulfide for self rate is 32 2 hydrogen atoms. So this comes out to be 104 plus 48 That's 1 52. So for cr 203, the Mueller must will be 52 into two because 52 is the molar mass of chromium plus 16 into three because 16 is the Mueller mask for oxygen. So first let us write the molar mass here at the top for each compound. So here we have been given the molar masses of the elements which are in youth here in this equation. So here, first we figure out The number of moles of this chromium three selfies. So we need to determine the number of moles of chromium trioxide which will be required. And it is given that the mass of chromium three cell fight that's produced Is 904 g. Chromium III Sulfate is non combustible.And this problem, we have been given that chromium trioxide is reacting with hydrogen sulfide to produce Chromium three cell fight and water.It is corrosive in nature and categorized as an irritant.It is very harmful to the environment and a major health hazard.Chromium III Sulfate is an inorganic ionic compound.

The percentages of individual elements of Cr 2(SO 4) 3 (dichromium trisulfate) are Cr - 26.52%, O - 48.95% and S - 24.53%.As seen in the structure of Chromium III Sulfate in the image above, the ratio of chromium to sulfate in this inorganic compound is 2:3.


 0 kommentar(er)
0 kommentar(er)
